 |
 |
|
|
|
 |
 |
|
|
|
June 24, 2024 - A Novel Broad-Spectrum Antiviral Against Influenza A Viruses, NV-387, Could Be an Important Weapon to Fight Bird Flu H5N1, Says NanoViricides
June 4, 2024 - This is NHP PK profile of NV-387 which showed desirable unusually slow decline
May 23, 2024 - NanoViricides Bolsters Partnership Efforts - Engages Aagami Inc
May 15, 2024 - NanoViricides Has Filed its Quarterly Report - NV-387 Advancing to Phase II Clinical Trial for the Treatment of RSV Infection
May 10, 2024 - NanoViricides to Participate in the 2024 EF Hutton Annual Global Conference On May 15 in New York City
April 30, 2024 - NanoViricides Reports that the Phase I NV-387 Clinical Trial is Completed Successfully and Data Lock is Expected Soon
February 15, 2024 - NanoViricides Has Filed its Quarterly Report - NV-387 Clinical Trial Healthy Subjects Part Successfully Completed, COVID Patient Treatment on the Horizon
January 4, 2024 - NanoViricides to Present at the Biotech Showcase in San Fransisco
November, 28 2023 - The Phase 1a/1b Human Clinical Trial of NV-CoV-2, the Company's Broad-Spectrum Antiviral Drug, Has Successfully Completed the First Part (Phase 1a), Reports NanoViricides
November, 15 2023 - NanoViricides Has Filed its Quarterly Report - NV-387 Broad-Spectrum Antiviral in Clinical Trials Has Additional Applications
November, 14 2023 - Broad-Spectrum Antiviral NV-387 (NV-CoV-2) in Phase 1a/1b Clinical Trial is Highly Effective in an Animal Model for MPox and Smallpox Drug Development
October, 11 2023 - NanoViricides, Inc. to Present at the Partnership Opportunities in Drug Delivery Conference in Boston on October 16, 2023 at 6:07pm ET
August, 21 2023 - The Phase 1a/1b Human Clinical Trial of NV-CoV-2, the Company's Broad-Spectrum Antiviral Drug, is Progressing Successfully, Reports NanoViricides
July 11, 2023 - Broad-Spectrum Antiviral NV-387 Has Demonstrated Excellent Effectiveness in RSV in a Lethal Lung Disease Animal Model
May 31, 2023 - NanoViricides, Inc. to Present at the BIO International Convention in Boston on June 5th, 2023 at 3:15pm ET
May 16, 2023 - NanoViricides Has Filed its Quarterly Report - Clinical Trials of Broad-Spectrum COVID Drug About to Begin
April 25, 2023 - NanoViricides is Expanding its Broad-Spectrum Antiviral Program to Explore Further Antiviral Applications of its COVID Clinical Drug Ingredient
April 17, 2023 - NanoViricides Has Shipped Drug Products for Impending Clinical Trials of NV-CoV-2, Its COVID Drug Candidate
Put cell phone in landscape to view |
|||||
NanoViricides Product Pipeline |
|||||
 |
 |
 |
 |
 |
 |
COVID |
RSV |
VZV "SHINGLES |
HSV -1 "GENITAL LESIONS" |
HSV-2 "COLD SORES" |
HIVCIDE |
Additional Virus Targets in R&D Stages |
|||||
 |
 |
 |
 |
 |
 |
SMALLPOX |
EBOLA, MARBURG, HEMORRHAGIC VIRUSES |
DENGUE VIRUS |
RABIES |
HEPATITIS C |
MANY MORE |

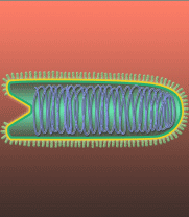
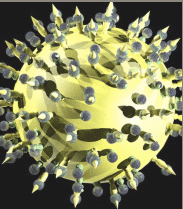
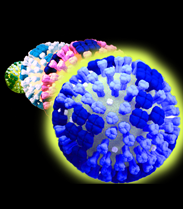
Imagine coursing through the blood stream, chasing through the body, grabbing and destroying the offending virus particles. NanoViricides, Inc. is striving to bring this imagination to fruition. A nanoviricide®, as defined by the Company, is a nanomachine that is armed to destroy a particular kind of virus. Which virus? That information is programmed into the nanoviricide, akin to the postal address on an envelope.
A "nanoviricide" is an agent designed by the Company to fool a virus into attaching to this antiviral nanomachine, in the same way that the virus normally attaches to the receptors on a cell surface. Once attached, the flexible nanoviricide glob wraps around the virus and traps it. In the process, the virus also loses its coat proteins that it needs to bind to a cell. The virus is thus neutralized and effectively destroyed. A nanoviricide completes the task of dismantling the virus particle without immune system assistance. This is the putative or designed mechanism of action of a nanoviricide. Thus nanoviricides represent the next great advance in "Immunotherapeutics" (antibodies and vaccines), which are currently well established antiviral strategies.
 |
Dr. Diwan invented novel polymeric micelle-based nanomedicine technologies
|
 |
Ms. Vyas was First Indian woman to be named CEO of a publicly listed US corporation
|
 |
Dr. Barton has over 25 years experience in drug discovery and development |
 |
Jay Tatake has over 25 years of experience in Organic Chemistry, Research and Process Development. |
|||
| Anil R. Diwan Executive Chairman, President |
Meeta Vyas Chief Financial Officer |
Randall Barton
Chief Scientific Officer - Consulting |
Jayant Tatake
Vice President, R&D |
|||||||
NanoViricides, Inc.
1 Controls Drive,
Shelton, CT 06484
Phone: +1-203-937-6137
Phone: +1-888-591-3579
Fax: +1-203-859-5095
E-mail: info@nanoviricides.com